Our Science
Doctor-Recommended
Impactful science-backed results by our Gut-X Axis formulas.
5+
Clinical Studies
15+
Exclusive Ingredients
20+
Global Patents
Pioneers of the ‘Gut-X Axis’
The physician-scientists behind res were among the first to sequence targeted restoration. By breaking down the fiber in the gut, we influence the dynamic connections between gut health and targeted bodily functions. This method allows us to deliver targeted benefits through our clinically- studied pre/pro/post-biotic supplements.
Our founder, Dr. Vivek Lal, is a double board-certified physician, an authority in NIH-funded microbiome research, and an experienced entrepreneur who previously launched Alveolus Bio, a company that develops FDA-approved pulmonary therapeutics. He also served as the Director of Clinical Innovation and Director of the Microbiome Lab at a top US research University. Together with our team of scientists, we’ve researched the microbiome's role in regulating respiratory pathways, and the first blend that scientifically supports lung structure and function through the Gut-Lung axis was developed: resB Lung Support Probiotic. Since then, the same team has developed new formulations for other health targets to be launched by res.*

C Vivek Lal, MD, FAAP
Founder & CEO, resbiotic
Quality Promise
We understand that not all pre/pro/post-biotics are created equally, and we’re setting a higher standard for our quality, research, and potent formulations. Our team’s scientific foundation ensures that our supplement development undergoes rigorous scientific analysis and testing, enabling us to deliver the highest-quality probiotic and prebiotic supplements.
Ingredient Sourcing
Our ingredient library and probiotic strains are maintained with proprietary deposits, all backed by live models (in vivo), human cellular laboratory research (in vitro and ex vivo), safety, and proof-of-concept data. In addition, all our formulas has undergone extensive clinical trials for safety and efficacy. Our blends are formulated by optimizing indicative biomarker responses in human tissue cells to optimize benefits across formulation.
Proprietary Formulas
Our globally patented or patent pending formulas contain ingredients produced in cGMP (Current Good Manufacturing Processes) environments by FDA-certified suppliers, specifically processed for extended stability and formula longevity. Our prebiotic, probiotic, and postbiotic ingredients and botanical extracts combine to create unique formulations tailored toward targeted systemic benefits, such as lung health, gut health, and metabolic support.
Production & Manufacturing
res blends are produced via blending and biofermentation by optimizing fermentation temperature, pH, mother culture purity, ingredient stability, and other factors. Ingredients are evaluated and precision blended to yield a consistent result every time. We manufacture in the USA under the most stringent quality checks.
Quality & Fulfillment
Every batch is analyzed for impurities and pathogens and precision blended according to current Good Manufacturing Practices (cGMP) from FDA-complaint manufacturing facilities. It is then sent to you from our FDA-registered fulfillment center. We only release supplements to the public that have met stringent quality, purity, and analytical testing requirements.
We’re third-party certified
We go above and beyond testing and certifying all our formulas to deliver the highest quality and clinically validated nutritional interventions for day-to-day health.


Proudly Non-GMO Project verified
resG prebeet ENERGY Prebiotic ingredients are Non-GMO Project verified, one of the most rigorous, independent, and trusted certifications for GMO avoidance. It validates that resG prebeet ENERGY Prebiotic is free of GMOs, or genetically modified organisms.
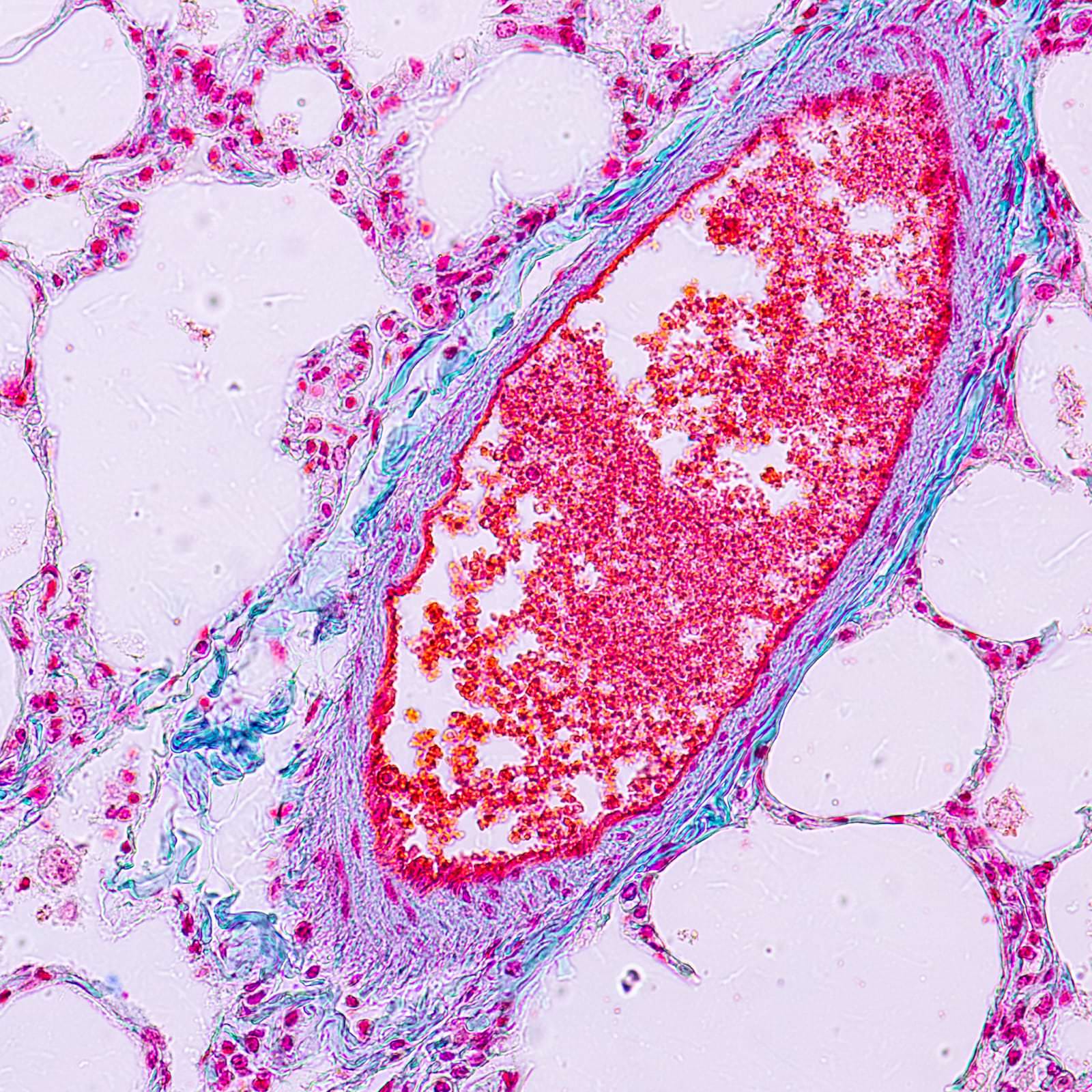
Heavy metal testing
Anything that comes from our soil, including our ingredients, has the potential to have natural heavy metal residue. At res, we test rigorously to ensure our pre/pro/post-biotics are free of heavy metals such as:
- Arsenic
- Cadmium
- Mercury
- Lead

Pesticide testing
At res, we test rigorously to ensure our pre/pro/post-biotic supplements are free of pesticides.
Microbiological analysis
Our supplements are always tested for the presence of harmful and disease microbes such as:
- E coli
- Staphylococcus
- Salmonella

Room temperature stability testing
Our supplements are tested by third party for prolonged stability and shelf life at various time points, so that you get consistent potency and results under room temperatures.


Diet inclusive, low FODMAP Monash certified
We’re glyphosate-residue-free and have a low FODMAP verified by Monash, the globally recognized symbol of trust in validating the highest standards of quality, accuracy, and compliance with FODMAP diet guidelines.

Prioritizing diversity, proudly kosher
Embracing our diverse community, ressupplements are certified kosher.

Proudly Non-GMO Project verified
resG prebeet ENERGY Prebiotic ingredients are Non-GMO Project verified, one of the most rigorous, independent, and trusted certifications for GMO avoidance. It validates that resG prebeet ENERGY Prebiotic is free of GMOs, or genetically modified organisms.
Heavy metal testing
Anything that comes from our soil, including our ingredients, has the potential to have natural heavy metal residue. At res, we test rigorously to ensure our pre/pro/post-biotics are free of heavy metals such as:
- Arsenic
- Cadmium
- Mercury
- Lead
Pesticide testing
At res, we test rigorously to ensure our pre/pro/post-biotic supplements are free of pesticides.
Microbiological analysis
Our supplements are always tested for the presence of harmful and disease microbes such as:
- E coli
- Staphylococcus
- Salmonella
Room temperature stability testing
Our supplements are tested by third party for prolonged stability and shelf life at various time points, so that you get consistent potency and results under room temperatures.

Diet inclusive, low FODMAP Monash certified
We’re glyphosate-residue-free and have a low FODMAP verified by Monash, the globally recognized symbol of trust in validating the highest standards of quality, accuracy, and compliance with FODMAP diet guidelines.
Prioritizing diversity, proudly kosher
Embracing our diverse community, ressupplements are certified kosher.







Clinical Results
Since 2009, we’ve been researching the role of the microbiome in regulating respiratory pathways. We’ve invested over a decade and countless hours into our formulas. We’re obsessed with the gut!
We approach supplement development by collaborating with the world’s leading scientists, research partners, and manufacturers across formula research, ingredient sourcing, clinical trials, supplement formulation, and third-party stability testing to deliver you the first (and only!) scientifically validated microbiome-based targeted restorative formulas.
95%
participants are likely to recommend resB to others.
resB Lung Support Probiotic
82%
participants reported quality of life improvement.
resB Lung Support Probiotic
400%
increase in GLP-1 levels in preclinical human cell studies.¹
resG prebeet ENERGY Prebiotic
200%+
increase in beneficial probiotics Bifidobacterium & Akkermansia which may boost GLP-1¹ and support GI struggles.*
resG prebeet ENERGY Prebiotic
300%
increase in GLP-1 hormone production.¹
resM GLP-1 Postbiotic
150%
increase in cellular glucose consumption.¹*
resM GLP-1 PostbioticresB® Lung Support Probiotic
1. Preclinical in vitro & ex vivo human cellular testing
Process: resB was tested for efficacy and benefits through live models.
Results: resB decreased markers of respiratory concerns, and the study described the mechanisms and efficacy of resB Lung Support Probiotic.
Peer-Reviewed Research Publication
2. Randomized open-label clinical trial
Process: Participants took resB Lung Support Probiotic blend twice daily for 30 days.
Results: Participants showed significant improvements in biomarkers and showed improvements measured by forced expiratory volume. Participants also noted an improvement in overall health, coughing, shortness of breath and breathing-related sleep disturbances. resB supplementation was found to be safe for consumption with almost no adverse side effects.
Peer-Reviewed Research Publication
3. Double-blind, randomized placebo-controlled clinical trial to evaluate quality of life
Process: Participants took resB twice daily for 3 months.
Results: Participants showed improvement and reported improvement in clinical status. resB supplementation was found to be safe for consumption with almost no adverse side effects.
resG prebeet® ENERGY Prebiotic
Three-arm randomized double-blind placebo-controlled trial for prebiotic-resistant starch
Process: A positive impact on gut bacteria and stool consistency was evaluated after 4-week clinical study through tracked stool samples against the Bristol Stool Form Scale.
Results: The prebiotic stimulated and increased beneficial health-associated bacteria, promoting a healthy metabolism, reducing diarrhea and constipation, and decreasing collagen breakdown.
Peer-Reviewed Research
Journal of Functional Foods Volume 108, September 2023
Front. Neurosci., 06 February 2023
1. Preclinical in vitro & ex vivo human cellular testing
Process: resB was tested for efficacy and benefits through live models.
Results: resB decreased markers of respiratory concerns, and the study described the mechanisms and efficacy of resB Lung Support Probiotic.
Peer-Reviewed Research Publication
2. Randomized open-label clinical trial
Process: Participants took resB Lung Support Probiotic blend twice daily for 30 days.
Results: Participants showed significant improvements in biomarkers and showed improvements measured by forced expiratory volume. Participants also noted an improvement in overall health, coughing, shortness of breath and breathing-related sleep disturbances. resB supplementation was found to be safe for consumption with almost no adverse side effects.
Peer-Reviewed Research Publication
3. Double-blind, randomized placebo-controlled clinical trial to evaluate quality of life
Process: Participants took resB twice daily for 3 months.
Results: Participants showed improvement and reported improvement in clinical status. resB supplementation was found to be safe for consumption with almost no adverse side effects.
Three-arm randomized double-blind placebo-controlled trial for prebiotic-resistant starch
Process: A positive impact on gut bacteria and stool consistency was evaluated after 4-week clinical study through tracked stool samples against the Bristol Stool Form Scale.
Results: The prebiotic stimulated and increased beneficial health-associated bacteria, promoting a healthy metabolism, reducing diarrhea and constipation, and decreasing collagen breakdown.
Peer-Reviewed Research
Journal of Functional Foods Volume 108, September 2023
Front. Neurosci., 06 February 2023
Our physician-scientist team

C. Vivek Lal, MD, FAAP
Founder & CEO

Dr. Bindiya Gandhi, MD
Medical Ambassador

Amit Gaggar, MD, PhD
Chief Medical Officer

Kara Siedman, RD, CDCES
Director of Partnerships

Thea Nicola, Md, PhD, MBA
Director, Lab Operations

Namasivayam Ambalavanan, MD, FAAP
Pediatric Lung Disease
Endowed Professor & Virginia Walker Chair in Pediatrics, University of Alabama at Birmingham

Yvonne Huang, MD
Microbiome, Adult Lung Disease
Associate Professor, Pulmonary & Critical Care Medicine & Director Huang Microbiome Lab, University of Michigan

Nirmal Sharma, MD
Lung Transplant
Assistant Professor Medical Director Lung Transplantation Program, Brigham & Women’s, Harvard University

Kent Willis, MD, FAAP
Gut-Lung Axis, Mycobiome
Assistant Professor & Director, Willis Lung Lab, University of Alabama at Birmingham

Mike Wells, MD
Clinical Trials, Adult Lung Disease
Associate Professor, Pulmonary & Critical Care Medicine, University of Alabama at Birmingham

Casey Morrow, PhD
Microbiome
Professor Emeritus, Microbiome Resource Center & Cell Development & Integrative Biology, University of Alabama at Birmingham
Answers to all your questions
What is the Gut-X Axis?
Our gut is our body's most immunologically active organ, communicating with different organ systems. What we consume in our gut acts as the entry point to several systemic connections. The gut-X axis denotes the axis or connections by which the gut communicates with various systemic organs or functions. The X could stand for the lungs, such as in the gut-lung axis, the heart in the gut-heart axis, the brain in the gut-brain axis, and so forth.
How long does it take to see benefits?
While people often report gut health benefits as early as their first week, systemic benefits can take longer. Some community members report breathing, circulatory, and energy benefits within six days, while others after 60-90 days. This variability can be attributed to many factors, including the importance of continuous daily use and the reality that some of us constantly think about how our bodies are feeling. In contrast, others go about their day without much thought.
How long until I get my order?
The turnaround time for standard shipping is within 3-5 business days. You may purchase expedited shipping if you need your order to arrive in 2 business days. If you are setting up a subscription order and require express shipping, please contact support@resbiotic.com or call us at 667-295-7372.
Keep in mind that packages can be marked delivered 24-48 hours before they are physically delivered. If you do not receive your package within 48 hours of being marked delivered, you can file a claim here or contact our Customer Experience team at support@resbiotic.com
What are my payment options? Can I use HSA/FSA?
We offer multiple payment avenues, including all major credit cards, Paypal, Apple Pay, Facebook Pay, and Google Pay.
Exciting news - we’ve partnered with TrueMed to allow you to use your Health Savings Account (HSA) or Flexible Spending Account (FSA). This means you can buy our supplements with pre-tax dollars, resulting in 30-40% net savings.
I need to modify my subscription; what do I do?
If you need to modify your subscription, please send us a message at support@resbiotic.com
Who is resB for?
Anyone 18+ who wants to prioritize their gut, lung, and immune health!
resB Lung Support Probiotic is a top physician-recommended and formulated supplement for continuous respiratory care. Customers use resB Lung Support Probiotic for respiratory structure and function support, cough relief, lung detox, and mucus relief.
Is resB Lung Support Probiotic safe to take with other supplements and medications for COPD, Asthma, etc?
While there are no known contraindications with resB Lung Support Probiotic and any over-the-counter or prescription medications, we encourage you to consult your primary care physician before adding new supplements to your routine.
Can I take resB Lung Support Probiotic for COPD, Bronchitis, Pulmonary Fibrosis, or Asthma? *
The resB Lung Support Probiotic formula is based on over 10 years of rigorous research to support healthy respiratory structure and function, healthy digestion, and the immune system while keeping everyone in mind. Our ingredients and supplements have been extensively studied in many settings, including physician-supervised preclinical and clinical trials in specific conditions.
That said, no supplement can make claims regarding any specific disease diagnosis. We recommend you check with your doctor or pharmacist before starting any new dietary supplement.
Can resB Lung Support Probiotic help smokers or people who vape?
This probiotic lung support was created with smokers and their lung health in mind. Our clinical trials also included smokers who developed lung problems such as asthma and COPD. We strongly encourage smokers to try quitting smoking to take control of their lung health. Also, no supplement can claim any specific conditions. We recommend you check with your doctor or pharmacist before starting any new dietary supplement.
What is GLP-1, and does resG prebeet increase GLP-1?
GLP-1 is Glucagon Like Peptide -1, a hormone that naturally delays gastric emptying and reduces appetite. GLP-1 has been made famous recently by Hollywood for weight loss functions.
resG prebeet has been shown to naturally increase GLP-1 in preclinical studies.¹
¹GLP-1 boost seen in human cellular studies.